Answer:
Q = 32,007.6 J
Step-by-step explanation:
Hello there!
In this case, since the energy involved during a heating/cooling process is:
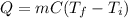
Thus, given the mass, specific heat of water, initial temperature and final one, we plug in obtain:
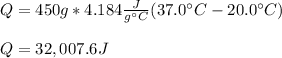
Best regards!