Answer:
236 cubic cm
Step-by-step explanation:
We are given that
Initial volume, V1=200 cubic centimeter
Initial temperature, T1=60 degree Celsius=60+273=333 K
Final temperature, T2=120 degree Celsius=120+273=393 K
Kelvin temperature=Celsius temperature+273
We have to find the new volume of the balloon.
We know that
Charles's law
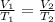
Using the formula
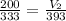
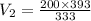
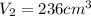
Hence, the new volume of the balloon will be 236 cubic cm.