Final Answer:
Yes, emission spectra have constraints on possible transitions, as they are governed by the quantized energy levels of electrons within atoms.
Step-by-step explanation:
Emission spectra result from the transition of electrons between different energy levels within an atom. According to quantum theory, electrons in atoms occupy discrete energy levels, and transitions between these levels emit or absorb specific amounts of energy. The constraints on possible transitions in emission spectra are dictated by the quantized nature of these energy levels. The allowed transitions correspond to the energy differences between these levels, following the equation
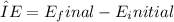 , where ΔE is the energy of the emitted photon, and
, where ΔE is the energy of the emitted photon, and
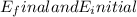 are the final and initial energy levels of the electron.
are the final and initial energy levels of the electron.
The quantization of energy levels in atoms leads to distinct emission lines in the spectra, each corresponding to a specific transition. For example, the Balmer series in hydrogen emission spectrum results from transitions to the n=2 energy level, while the Lyman series corresponds to transitions to the n=1 level. The constraints on transitions are instrumental in identifying elements through their unique emission spectra.
Deviations from these constraints would violate the conservation of energy and are not observed in the emission spectra of elements. In summary, the quantized nature of energy levels imposes strict constraints on the transitions that contribute to emission spectra, providing a fundamental understanding of atomic structure and behavior.