Final answer:
The atomic mass of an element is quantified using units such as Daltons and moles. The atomic mass unit is defined in relation to carbon-12. The molar mass of a substance is the mass in grams of one mole of representative particles.
Step-by-step explanation:
The atomic mass of an element is quantified using units such as Daltons and moles. The atomic mass unit (amu) is defined as one twelfth the mass of an atom of carbon-12 and is used as a reference standard. For example, the atomic mass of helium-4 is 4.0026 amu. On the other hand, the molar mass of an element or compound is the mass in grams of one mole of representative particles of that substance. The mole is a counting unit that links the mass in grams to the number of atoms or molecules, and is equal to
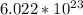 entities.
entities.