The number of atoms present in 42.10 grams of bromine gas, Br₂ is
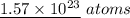
How to calculate the number of atoms of bromine gas, Br₂?
The number of atoms present in 42.10 grams of bromine gas, Br₂ can be calculated as shown below:
- Mass of bromine gas, Br₂ given (m) = 42.10 grams
- Molar mass of bromine gas, Br₂ (M) = 159.808 g/mol
- Mole of bromine gas, Br₂ present = m / M = 42.10 / 159.808 = 0.26 mole
- Avogadro's constant =
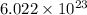
- Number of atoms of bromine gas, Br₂ present =?
Number of atoms of bromine gas, Br₂ present = Mole × Avogadro's constant
= 0.26 ×
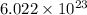 =
=
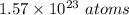
From the above calculation, we can conclude that the number of atoms present in 42.10 grams of bromine gas, Br₂ is
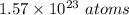
Complete question:
How many atoms are present in 42.10 g of bromine gas?
42.10 g of Br₂ = ____atoms