Answer:
28 g CO
Step-by-step explanation:
First convert grams to moles.
1 mole C = 12.011 g (I'm just going to round to 12 for the sake of this problem)
12 g C •
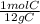 = 1 mol C
= 1 mol C
1 mol O = 15.996 g (I'm just going to round to 16)
16 g O •
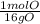 = 1 mol O
= 1 mol O
So the unbalanced equation is:
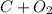 ->
->
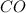 (the oxygen has a 2 subscript because it is part of HONClBrIF meaning when not in a compound these elements appear in pairs - called diatomic elements)
(the oxygen has a 2 subscript because it is part of HONClBrIF meaning when not in a compound these elements appear in pairs - called diatomic elements)
The balanced equation is:
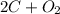 ->
->
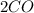
However, carbon is the limiting reactant in this equation and two moles cannot react because only 12 g (1 mole) are present. Therefore, use the equation
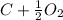 ->
->
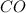 .
.
1 mole of CO is formed, therefore 12 g + 16 g = 28 g CO.