Answer:
+0.8M/s
Step-by-step explanation:
We are given that
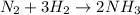
Rate of reacting of N2, d/dt([N2])=-0.4M/s
We have to find the rate by which NH3 is being produced.
We know that
![-(d[N_2])/(dt)=(1)/(2)(d[NH_3])/(dt)](https://img.qammunity.org/2022/formulas/advanced-placement-ap/high-school/226z8puehjc4x4i034wmiip5fk6c0tx9ug.png)
Using the formula
![-(-0.4)=(1)/(2)(d[NH_3])/(dt)](https://img.qammunity.org/2022/formulas/advanced-placement-ap/high-school/re9roxu0h06z72lvutw82obwdi6famab7j.png)
![(d[NH_3])/(dt)=0.4* 2](https://img.qammunity.org/2022/formulas/advanced-placement-ap/high-school/g8vctwn2hzclwwlookufwrqm6gexpubf4j.png)
![(d[NH_3])/(dt)=0.8M/s](https://img.qammunity.org/2022/formulas/advanced-placement-ap/high-school/pe25a65jz8bbkber85kju8ksu7yigsj9k0.png)
Hence, the rate by which NH3 is being produced=0.8M/s
Option C is correct.
0.8M/s