Answer:
C. 12.8 liters.
Step-by-step explanation:
The Standard Temperature and Pressure (STP) of a gas are 273.15 K and 100 kilopascals. From Avogadro's Law, a mole of carbon dioxide contains
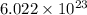 molecules. If we suppose that carbon dioxide behaves ideally, then the equation of state for ideal gas is:
molecules. If we suppose that carbon dioxide behaves ideally, then the equation of state for ideal gas is:
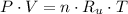 (1)
(1)
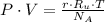 (1b)
(1b)
Where:
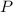 - Pressure, measured in pascals.
- Pressure, measured in pascals.
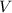 - Volume, measured in liters.
- Volume, measured in liters.
 - Amount of molecules, no unit.
- Amount of molecules, no unit.
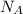 - Avogadro's number, no unit.
- Avogadro's number, no unit.
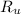 - Ideal gas constant, measured in pascal-liters per mole-Kelvin.
- Ideal gas constant, measured in pascal-liters per mole-Kelvin.
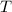 - Temperature, measured in Kelvin.
- Temperature, measured in Kelvin.
If we know that
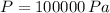 ,
,
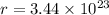 ,
,
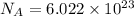 ,
,
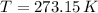 and
and
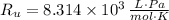 , then the volume of carbon dioxide at STP is:
, then the volume of carbon dioxide at STP is:
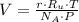
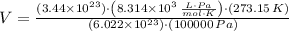
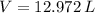
Therefore, the correct answer is C.