The expression for the final work done is given by W = (constant/
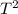 )(Vf - Vi), where constant is a constant value, T is the temperature, and Vi and Vf are the initial and final volumes, respectively.
)(Vf - Vi), where constant is a constant value, T is the temperature, and Vi and Vf are the initial and final volumes, respectively.
The equation given,
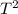 P = constant, represents a reversible transformation of a gas. To find the expression for the final work done, we can use the ideal gas law and the concept of work done by a gas.
P = constant, represents a reversible transformation of a gas. To find the expression for the final work done, we can use the ideal gas law and the concept of work done by a gas.
First, let's rearrange the given equation to solve for pressure (P) in terms of temperature (T):
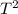 P = constant
P = constant
P = constant/
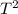
Next, let's consider the ideal gas law, which states:
PV = nRT
In this case, the mass of the gas (m) is given, so we can rewrite the ideal gas law as:
PV = (m/M)RT
where M is the molar mass of the gas.
Now, we can substitute the expression for pressure (P) from the rearranged equation into the ideal gas law:
(constant/
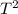 )V = (m/M)RT
)V = (m/M)RT
To find the final work done, we need to calculate the difference between the initial and final volumes. Let's assume the initial volume is Vi and the final volume is Vf.
The work done by a gas can be expressed as:
W = PΔV
where ΔV is the change in volume.
Using the ideal gas law, we can express the change in volume as:
ΔV = (m/M)RTf - (m/M)RTi
Substituting the expression for pressure (P) into the work equation and simplifying, we get:
W = (constant/
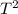 )(Vf - Vi)
)(Vf - Vi)
In summary, the expression for the final work done is:
W = (constant/
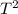 )(Vf - Vi)
)(Vf - Vi)