Determine thermodynamic parameters, processes, and quantities for the given refrigerator cycle, including CV, CP, state points, work, heat, and the performance coefficient. Additional data may be required for a complete analysis.
To determine the thermodynamic parameters and quantities for the given refrigerator cycle, we need to follow the processes described in the figure and apply the appropriate formulas.
1. CV (Specific Heat at Constant Volume):
The specific heat at constant volume (CV) is calculated using the ideal gas law and the relationship
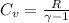 , where (R) is the gas constant and
, where (R) is the gas constant and
 is the heat capacity ratio.
is the heat capacity ratio.
2. CP (Specific Heat at Constant Pressure):
For diatomic hydrogen,
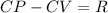 , and thus CP = CV + R. This relationship is derived from the first law of thermodynamics.
, and thus CP = CV + R. This relationship is derived from the first law of thermodynamics.
3. State Point P (a):
Given values are
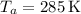 ,
,
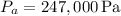 , and
, and
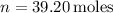 . Use the ideal gas law PV = nRT to calculate the volume
. Use the ideal gas law PV = nRT to calculate the volume
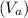 at point a.
at point a.
4. Process a→b:
During this process, the pressure quadruples
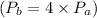 . Use the ideal gas law to find the new temperature
. Use the ideal gas law to find the new temperature
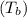 , and calculate the work
, and calculate the work
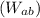 and heat
and heat
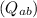 using thermodynamic relations.
using thermodynamic relations.
5. Process b→c:
The figure does not provide enough information for a direct calculation of
 . Additional data or assumptions are needed to proceed with the analysis.
. Additional data or assumptions are needed to proceed with the analysis.
6. Process c→a:
Similar to process b→c, additional information is required for a direct calculation of
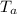 .
.
7. Total Heat Absorbed and Released (Qhot and Qcold):
Determine the total heat absorbed
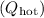 and released
and released
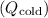 during the entire cycle.
during the entire cycle.
8. Performance Coefficient:
Calculate the performance coefficient using the formula
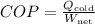 .
.
Given the nature of the refrigerator cycle and the lack of complete information, assumptions or additional data might be necessary for a comprehensive analysis.