Answer:
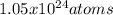
Step-by-step explanation:
Hello!
In this case, according to the definition of the Avogadro's number, it is possible to realize that one molecule of CO2 contains 3 moles of atoms, one of carbon and two of oxygen, thus, we have:
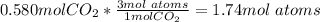
Then, we use the Avogadro's number to obtain:
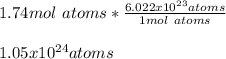
Best regards!