At the end of exactly one day, there will be approximately 5.01 grams of tungsten-187 remaining, given the half-life of 23.9 hours.
The decay of a radioactive substance follows an exponential decay equation given by:
![\[ N(t) = N_0 * \left((1)/(2)\right)^{\frac{t}{T_{\text{1/2}}}} \]](https://img.qammunity.org/2024/formulas/chemistry/college/ypktsfw6o6epc2jifzis2jba34c5dyat48.png)
where:
-
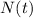 is the quantity of the substance at time
is the quantity of the substance at time
 ,
,
-
 is the initial quantity of the substance,
is the initial quantity of the substance,
-
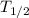 is the half-life of the substance.
is the half-life of the substance.
In this case, for tungsten-187,
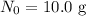 and
and
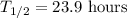 . After one day
. After one day
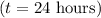 , we can substitute these values into the equation:
, we can substitute these values into the equation:
![\[ N(24) = 10.0 * \left((1)/(2)\right)^{(24)/(23.9)} \]](https://img.qammunity.org/2024/formulas/chemistry/college/jmm8bbm26ujtjazir0a1f6q89ewmjp41ky.png)
Calculating the quantity of tungsten-187
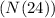 at the end of exactly one day using the exponential decay equation:
at the end of exactly one day using the exponential decay equation:
![\[ N(24) = 10.0 * \left((1)/(2)\right)^{(24)/(23.9)} \]](https://img.qammunity.org/2024/formulas/chemistry/college/jmm8bbm26ujtjazir0a1f6q89ewmjp41ky.png)
![\[ N(24) = 10.0 * \left((1)/(2)\right)^(1.00209) \]](https://img.qammunity.org/2024/formulas/chemistry/college/749k94wbf3py044wxbx6e4t9fljq5no5b7.png)
![\[ N(24) \approx 10.0 * 0.500786 \]](https://img.qammunity.org/2024/formulas/chemistry/college/47yjl1ys60gl2ci6tuaimtu7efjs4xyhsz.png)
![\[ N(24) \approx 5.00786 \, \text{g} \]](https://img.qammunity.org/2024/formulas/chemistry/college/nllkkrhpcw8j6jpxzbv83h32alh0bdagch.png)
So, at the end of exactly one day, there will be approximately 5.01 grams of tungsten-187 remaining, given the half-life of 23.9 hours.