The kinetic energy of a particle with a mass of
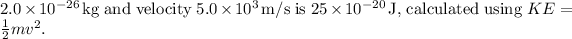
To find the kinetic energy (KE) of the particle, you can use the formula:
![\[ KE = (1)/(2)mv^2 \]](https://img.qammunity.org/2024/formulas/physics/high-school/vff65mwlyttobps790tmf5z1vevvg1n24b.png)
where:
-
 is the mass of the particle,
is the mass of the particle,
-
 is the velocity of the particle.
is the velocity of the particle.
Given:
-
 (mass of the particle),
(mass of the particle),
-
 (velocity of the particle).
(velocity of the particle).
Plug in these values into the formula:
![\[ KE = (1)/(2) * (2.0 * 10^(-26) \, \text{kg}) * (5.0 * 10^3 \, \text{m/s})^2 \]](https://img.qammunity.org/2024/formulas/chemistry/college/mzfrs2truyluvd0kpasxth0x1bqw222ymh.png)
Calculate the result:
![\[ KE = (1)/(2) * (2.0 * 10^(-26)) * (25 * 10^6) \, \text{J} \]](https://img.qammunity.org/2024/formulas/chemistry/college/e2t78mhrs4y624sg9kzzo9dpnwf8v0kgsx.png)
![\[ KE = 0.5 * (2.0 * 10^(-26)) * (25 * 10^6) \, \text{J} \]](https://img.qammunity.org/2024/formulas/chemistry/college/mlt0d4u9r7nwshel1yw0ntrjg3fhkqexuo.png)
![\[ KE = 25 * 10^(-26) * 10^6 \, \text{J} \]](https://img.qammunity.org/2024/formulas/chemistry/college/muf8xlx06hy1944jckqh4h6yy0k8r4ms5v.png)
![\[ KE = 25 * 10^(-20) \, \text{J} \]](https://img.qammunity.org/2024/formulas/chemistry/college/13gkang3drdnxqy3v7vdjioya163q445q7.png)
So, the kinetic energy of the particle is
THE probable question maybe:
28. A particle with a mass of 2.0 × 10^-26 kg is moving with a velocity of 5.0 × 10^3 m/s. Determine the kinetic energy of the particle using the formula \( KE = \frac{1}{2}mv^2 \). Given that Planck's constant is \( h = 6.62 \times 10^{-34} \, J \cdot s \).
(Note: You can use this information to calculate the kinetic energy of the particle.)