Answer:

Step-by-step explanation:
To convert from moles to grams, we must use the molar mass.
Recall that water's molecular formula is H₂O. It contains hydrogen and oxygen. Look up the two elements masses on the Periodic Table.
- Hydrogen (H): 1.008 g/mol
- Oxygen (O): 15.999 g/mol
Now, use these masses to find water's mass. The subscript of 2 tells us there are 2 atoms of hydrogen, so we multiply hydrogen's mass by 2 and add oxygen's.
- H₂O= 2(1.008 g/mol) + 15.999 g/mol = 18.015 g/mol
Use the molar mass as a ratio.
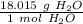
Multiply by the given number of moles.
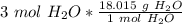
The moles of water will cancel.
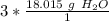
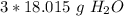
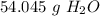
Round to the nearest whole number. The 0 in the tenth place tells us to leave the number as is.
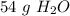
There are about 54 grams of water in 3 moles.