Answer: The mass of
 and
and
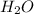 produced are 336.6 g and 183.6 g respectively.
produced are 336.6 g and 183.6 g respectively.
Step-by-step explanation:
The combustion reaction between propane and oxygen leads to formation of carbon dioxide and water.
Law of Conservation of mass states that the mass will remain constant for a balanced equation. This is carried out when the total number of atoms on reactant side is same as the total number of atoms on the product side. Thus the equation must be balanced.
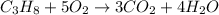
a) 1 mol of propane produces = 3 moles of

Thus 2.55 mol of propane produces =
![(3)/(1)* 2.55=7.65 moles of [tex]CO_2](https://img.qammunity.org/2022/formulas/chemistry/college/r0jku1wckfd1yiog4cjvv84bz1slsbpo01.png)
mass of
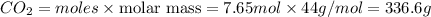
b) 1 mol of propane produces = 4 moles of
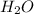
Thus 2.55 mol of propane produces =
![(4)/(1)* 2.55=10.2 moles of [tex]H_2O](https://img.qammunity.org/2022/formulas/chemistry/college/7ns665tsvutmg4kd49nf3i9xmapu1rss58.png)
mass of
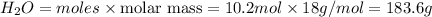
The mass of
 and
and
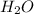 produced are 336.6 g and 183.6 g respectively.
produced are 336.6 g and 183.6 g respectively.