Answer:
5.00 mol O₂
Step-by-step explanation:
In order to solve this problem we will need to convert moles of water to moles of oxygen. We will do that using the stoichiometric coefficients, as follows:
- 10.0 mol H₂O *
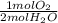 = 5.00 mol O₂
= 5.00 mol O₂
Thus 5.00 moles of oxygen would be produced from 10.0 moles of water.