Answer:
MM = 182.13g/mol
Step-by-step explanation:
Hello!
In this case, since the boiling point elevation is computed via:
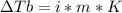
Thus, since adrenaline is nonionizing, we can compute the molality at first instance:
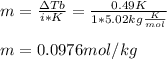
Now, we compute the the moles of adrenaline in 360 g (0.360 kg) of CCl4:
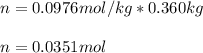
Finally, the molar mass:
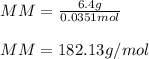
Best regards!