Final answer:
The Mach numbers: At the Inlet (a):
![\[(T_2)/(T_1) = \left(1 + (\gamma - 1)/(2)M_{\text{inlet}}^2\right)\]](https://img.qammunity.org/2024/formulas/engineering/college/5w4dy820xxl9eocep2h54lke2ejydx7bu3.png) Solve for
Solve for
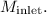
At the Exit (b):
![\[(T_2)/(T_1) = \left(1 + (\gamma - 1)/(2)M_{\text{exit}}^2\right)\]](https://img.qammunity.org/2024/formulas/engineering/college/dyslral18gbpfa42zxrxputr47xfpvwp2n.png) Solve for
Solve for
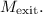
Step-by-step explanation:
To find the Mach numbers at the inlet and exit of the adiabatic nozzle, we can use the isentropic relations for ideal gases. The isentropic relations relate the Mach number M , temperature T , and other properties across a nozzle or diffuser.
The isentropic relations for an ideal gas are as follows:
1. Isentropic relation for temperature and Mach number:
![\[(T_2)/(T_1) = \left(1 + (\gamma - 1)/(2)M^2\right)\]](https://img.qammunity.org/2024/formulas/engineering/college/lhg7pbu091lv34y9vjn9liz2cnq50y4de6.png)
where:
-
 is the initial temperature (in Kelvin) at the inlet,
is the initial temperature (in Kelvin) at the inlet,
-
 is the final temperature (in Kelvin) at the exit,
is the final temperature (in Kelvin) at the exit,
-
 is the ratio of specific heats
is the ratio of specific heats
 ,
,
-
 is the Mach number.
is the Mach number.
2. Isentropic relation for velocity and Mach number:
![\[(V_2)/(V_1) = (1)/(M)\sqrt{(T_2)/(T_1)\left((\gamma + 1)/(2) + (\gamma - 1)/(2)M^2\right)}\]](https://img.qammunity.org/2024/formulas/engineering/college/mhsh7gk9yfg2qsfvebklzq1qe4ofcdehcf.png)
where:
-
 is the initial velocity at the inlet,
is the initial velocity at the inlet,
-
 is the final velocity at the exit.
is the final velocity at the exit.
Given:
-
 (initial temperature at the inlet),
(initial temperature at the inlet),
-
 (final temperature at the exit),
(final temperature at the exit),
-
 (initial velocity at the inlet),
(initial velocity at the inlet),
-
 (ratio of specific heats for carbon dioxide).
(ratio of specific heats for carbon dioxide).
Now, let's calculate the Mach numbers:
At the Inlet (a):
![\[(T_2)/(T_1) = \left(1 + (\gamma - 1)/(2)M_{\text{inlet}}^2\right)\]](https://img.qammunity.org/2024/formulas/engineering/college/5w4dy820xxl9eocep2h54lke2ejydx7bu3.png)
Solve for
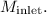
At the Exit (b):
![\[(T_2)/(T_1) = \left(1 + (\gamma - 1)/(2)M_{\text{exit}}^2\right)\]](https://img.qammunity.org/2024/formulas/engineering/college/dyslral18gbpfa42zxrxputr47xfpvwp2n.png)
Solve for
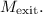
These equations are non-linear, and you may need to use numerical methods or specialized software to find the Mach numbers accurately. If you have access to engineering software or online calculators, they can provide a quick solution.