Answer:
118.61 mL.
Step-by-step explanation:
Hello!
In this case, since we know the concentration in the medication:
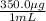
Now, since there are 0.50 mg of the medication per kilogram of patient, we can compute the volume as shown below for a 183-lb patient:
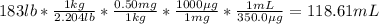
Best regards!