Answer:
Very dense.
Step-by-step explanation:
Conceptually, you are taking a large amount of atoms and putting them into a small container. This means the spaces between atoms has to be smaller and the substance is more dense. (see image)
Mathematically, the equation for density is mass divided by volume (
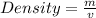 ). If you divide a big number by a small number, you still have a pretty big number (ex 4/2=2 versus 6/1=6)
). If you divide a big number by a small number, you still have a pretty big number (ex 4/2=2 versus 6/1=6)