Fraction of monomer remaining: 0.9867
Fraction of initiator remaining: 0.8264
Peroxide Decomposition and Monomer Polymerization
Here's how to calculate the remaining fractions of monomer and initiator in the system:
1. Peroxide Decomposition:
We know the decomposition is first-order, with 20% decomposition in 60 minutes. This translates to a rate constant (k_p) of:
k_p = ln(1 - 0.2) /
 ≈ 0.0115
≈ 0.0115
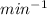
2. Initial Rate of Polymerization:
With an initial initiator concentration (P_0) of 4.0x10^-4 mol/L and the given system parameters (kp2/kt and f), we can calculate the initial rate of polymerization (Rp):
Rp = k p2 * f *
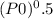
 ≈ 0.0013 mol/L*min
≈ 0.0013 mol/L*min
3. Monomer Conversion and Remaining Fraction:
During the 10 minutes reaction time, the amount of monomer consumed (Xc) is:
Xc = Rp * t / P0 ≈ 0.013 mol/L
The fraction of monomer remaining (X monomer) is:
X monomer = 1 - Xc ≈ 0.9867
4. Peroxide Remaining Fraction:
After 10 minutes, the remaining fraction of peroxide (X peroxide) is:
X peroxide = P remaining / P 0 = exp(-k p * t) ≈ 0.8264
Therefore:
Fraction of monomer remaining: 0.9867
Fraction of initiator remaining: 0.8264