The density of the unknown liquid is approximately
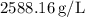 when 25.00 mL is transferred into the flask.
when 25.00 mL is transferred into the flask.
To find the density of the unknown liquid, we can use the formula:
![\[ \text{Density} = \frac{\text{Mass}}{\text{Volume}} \]](https://img.qammunity.org/2022/formulas/chemistry/college/y4q0ersj1vz20d6l1q18u5zvbgy89ojz64.png)
The mass of the liquid is the difference between the mass of the flask plus the liquid and the mass of the empty flask:
![\[ \text{Mass of liquid} = \text{Mass of flask + liquid} - \text{Mass of empty flask} \]](https://img.qammunity.org/2022/formulas/chemistry/college/xs0ir3248ndlnb2ig8pt0uxkn0qqmgxsz6.png)
![\[ \text{Mass of liquid} = 143.246 \, \text{g} - 78.542 \, \text{g} \]](https://img.qammunity.org/2022/formulas/chemistry/college/u30nlo79ppzb368a0as4l47by9c5n7ordj.png)
![\[ \text{Mass of liquid} = 64.704 \, \text{g} \]](https://img.qammunity.org/2022/formulas/chemistry/college/phqc9iux639tffwsl62qvbgb8it9vivtay.png)
The volume of the liquid is given as 25.00 mL. However, it's crucial to convert this volume to liters for consistency in units. There are 1000 milliliters in a liter, so:
![\[ \text{Volume of liquid} = 25.00 \, \text{mL} * (1)/(1000) \, \text{L/mL} \]](https://img.qammunity.org/2022/formulas/chemistry/college/7a87trinysawx0kqmom27dxs61lmeh1mj8.png)
![\[ \text{Volume of liquid} = 0.025 \, \text{L} \]](https://img.qammunity.org/2022/formulas/chemistry/college/ho5yrtte1cmiendnv9n1lqmiupzzq4tb5h.png)
Now, apply the formula for density:
![\[ \text{Density} = \frac{\text{Mass of liquid}}{\text{Volume of liquid}} \]](https://img.qammunity.org/2022/formulas/chemistry/college/mjvykvzv83mx4wphr5mubtstkg68zepvss.png)
![\[ \text{Density} = \frac{64.704 \, \text{g}}{0.025 \, \text{L}} \]](https://img.qammunity.org/2022/formulas/chemistry/college/649jmww8kn96pbuys8mzmb2ttwloc2eacv.png)
![\[ \text{Density} \approx 2588.16 \, \text{g/L} \]](https://img.qammunity.org/2022/formulas/chemistry/college/yqdctze31o45pv5i23m1brz8wxu2rvcm7s.png)
Therefore, the density of the unknown liquid is approximately
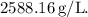
The question probable maybe:
A student finds the mass of an empty flask to be 78.542 grams. She carefully transfers 25.00 mL of an unknown liquid into the flask and the mass of the flask plus the liquid together is 143.246 grams. What is the density of this unknown liquid?