The correct answer is: has 3 valence electrons. The correct answer is option A.
The correct answer is A. has 3 valence electrons. Carbon actually has 4 valence electrons, which allows it to form strong covalent bonds with other atoms. This ability to form multiple bonds is one of the unique properties of carbon.
For example, carbon can form single bonds with 4 different atoms, such as in methane (
 ). In this molecule, carbon forms 4 single bonds with 4 hydrogen atoms.
). In this molecule, carbon forms 4 single bonds with 4 hydrogen atoms.
Carbon can also form double bonds with other atoms, as in ethene (
 ). In this molecule, carbon forms a double bond with another carbon atom, resulting in a strong and stable structure.
). In this molecule, carbon forms a double bond with another carbon atom, resulting in a strong and stable structure.
Additionally, carbon can also form rings, as seen in compounds such as benzene
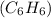 or cyclohexane
or cyclohexane
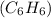 . These ring structures provide stability and contribute to the diversity of carbon compounds found in nature.
. These ring structures provide stability and contribute to the diversity of carbon compounds found in nature.
Therefore, all the properties mentioned in options B, C, and D are actually true for carbon, making option A the correct answer.