Answer:
0 J
Step-by-step explanation:
The change in internal energy of the system can be determined using the first law of thermodynamics, which is expressed as:
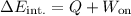
Where:
- 'ΔE_int.' is the change in internal energy.
- 'Q' is the heat added to the system (if the system loses heat, this value is negative).
- 'W_on' is the work done on the system (if the system does work, this value is negative).
In our case:
- The system loses 250 J of heat to the environment, so Q = −250 J.
- The environment does 250 J of work on the system, so W_on = +250J.
Plug in our values:
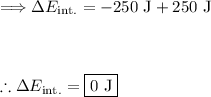
The change in internal energy of the system is 0 J. This means that the internal energy of the system remains unchanged.