Answer:
784500 Joules
Step-by-step explanation:
To start, you will need an equation to find the change in energy. This can be found by using
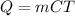 or by using integrated heat capacities.
or by using integrated heat capacities.
to find the mass of water,
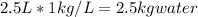
1kg/L is the density of water.
Then,
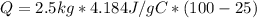
Notice that all of the units do not cancel out properly, so some unit conversions are needed to find the correct answer.