Answer:
the work done on the gas is 4,988.7 J.
Step-by-step explanation:
Given;
number of moles of the monoatomic gas, n = 4 moles
initial temperature of the gas, T₁ = 300 K
final temperature of the gas, T₂ = 400 K
The work done on the gas is calculated as;
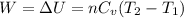
For monoatomic ideal gas:
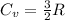
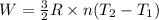
Where;
R is ideal gas constant = 8.3145 J/K.mol
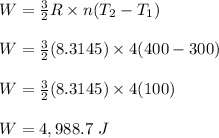
Therefore, the work done on the gas is 4,988.7 J.