Answer:
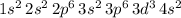 .
.
Step-by-step explanation:
Since the atomic number of vanadium is
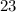 , there would be
, there would be
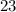 electrons in this atom.
electrons in this atom.
The maximum number of electrons in each subshell (
 ,
,
 ,
,
 , etc.) is:
, etc.) is:
 for each
for each
 subshell (
subshell (
 ,
,
 ,
,
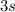 , etc.),
, etc.),
 for each
for each
 subshell (
subshell (
 ,
,
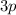 , etc.,) and
, etc.,) and
 for each
for each
 subshell (e.g.,
subshell (e.g.,
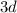 .)
.)
By the Aufbau Principle, the first few electron subshells are filled in the following order:
 ,
,
 ,
,
 ,
,
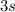 ,
,
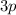 ,
,
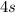 ,
,
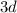 .
.
To find the electron configuration of Vanadium, start by filling the subshells in the order from the Aufbau Principle. Keep track of the total number of electrons that be placed so far:
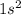 (the
(the
 subshell is now full,
subshell is now full,
 electrons placed in total.)
electrons placed in total.)
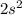 (
(
 electrons in total.)
electrons in total.)
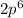 (
(
 electrons in total.)
electrons in total.)
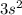 (
(
 electrons in total.)
electrons in total.)
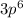 (
(
 electrons in total.)
electrons in total.)
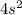 (
(
 electrons in total.)
electrons in total.)
By the Aufbau Principle, the next subshell would be
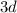 , which holds up to
, which holds up to
 electrons. However, since there are only
electrons. However, since there are only
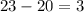 more electrons to be placed, the
more electrons to be placed, the
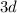 subshell of this atom will not be entirely filled:
subshell of this atom will not be entirely filled:
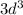 .
.
The resultant electron configuration of this atom would be:
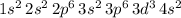 .
.