Answer: a. +2, cation and magnesium ion .
b. -1, anion, chloride
c. -2, anion, oxide
d. +1. cation , potassium ion
Step-by-step explanation:
When an atom accepts an electron negative charge is created on atom and is called as anion.
When atom loses an electron positive charge is created on atom and is called as cation.
Magnesium (Mg) with atomic number of 12 has electronic configuration of 2,8,2 and thus it can lose 2 electrons to form
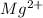 cation and becomes magnesium ion.
cation and becomes magnesium ion.
Chlorine (Cl) with atomic number of 17 has electronic configuration of 2,8,7 and thus it can gain 1 electron to form
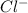 anion and becomes chloride.
anion and becomes chloride.
Oxygen (O) with atomic number of 8 has electronic configuration of 2,6 and thus it can gain 2 electrons to form
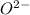 anion and becomes oxide.
anion and becomes oxide.
Potassium (K) with atomic number of 19 has electronic configuration of 2,8,8,1 and thus it can lose 1 electron to form
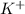 cation and becomes potassium ion.
cation and becomes potassium ion.