Answer
Step-by-step explanation:
Magnesium Oxide has a formula of MgO.
Magnesium has a molar mass of 24.30g/mol and Oxygen has a molar mass of 15.99g/mol.
From this, we can say we are assuming a 1 mole sample of magnesium oxide, as the stoichiometric coefficients will always be the same, as will the ratio of oxygen to magnesium.
From this,
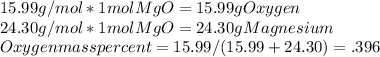
The Magnesium equation will be the same but a different numerator. Notice, if there were different coefficients on the elements, you would have to multiply by the stoich coefficient.