Answer:
P = 84.1 MPa
Step-by-step explanation:
- The pressure at the bottom of column of of salt water of height h, is given by the following expression:
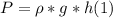
where ρ = density of salt water (in Kg/m³),
g = acceleration due to gravity (in m/s²)
h = height of the column of water.
- Replacing by their values in (1):
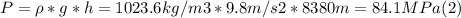
- Neglecting the atmospheric pressure, the pressure on the cube at the bottom of Puerto Rico Trench is given by (2):
- P = 84.1 MPa.