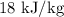Answer:
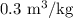
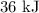
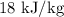
Step-by-step explanation:
V = Volume of air =
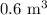
P = Power = 10 W
t = Time = 1 hour
m = Mass of air = 2 kg
Specific volume is given by
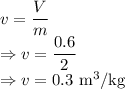
The specific volume at the final state is
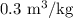
Work done is given by
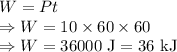
The energy transfer by work, is
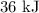
Change in specific internal energy is given by
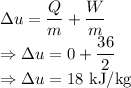
The change in specific internal energy of the air is