Answer:
The balanced molecular equation for the reaction is:
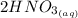 +
+
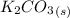 →
→
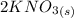 +
+
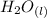 +
+
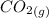 ↑
↑
Step-by-step explanation:
The weak nitric acid reacts with the strong potassium base to produce salt, water and carbon dioxide which is given off.
Molecular ratio of reactants is 2 : 1.
All phases are represented in the equation: aq for aqueous, l for liquid and g for gas (notice how they are written in subscript).
I hope this was helpful.