Answer: 15.0 g of
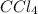 will be formed upon the complete reaction of 27.7 grams of chlorine gas with excess carbon disulfide
will be formed upon the complete reaction of 27.7 grams of chlorine gas with excess carbon disulfide
Step-by-step explanation:
To calculate the moles :
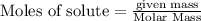
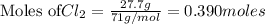
The balanced chemical reaction is:
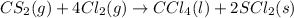
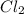 is the limiting reagent as it limits the formation of product and
is the limiting reagent as it limits the formation of product and
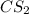 is the excess reagent.
is the excess reagent.
According to stoichiometry :
4 moles of
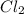 give= 1 mole of
give= 1 mole of
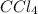
Thus 0.390 moles of will give =
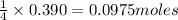 of
of
Mass of
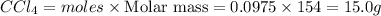
Thus 15.0 g of
 will be formed upon the complete reaction of 27.7 grams of chlorine gas with excess carbon disulfide
will be formed upon the complete reaction of 27.7 grams of chlorine gas with excess carbon disulfide