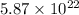 molecules of water is approximately
molecules of water is approximately
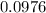 moles of water.
moles of water.
To determine the number of moles in a given quantity of molecules, you can use Avogadro's number, which is approximately
 molecules/mol.
molecules/mol.
Given that you have
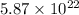 molecules of water, you can use the following formula:
molecules of water, you can use the following formula:
![\[\text{Number of moles} = \frac{\text{Number of molecules}}{\text{Avogadro's number}}\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/o22mdm4vawlx3ytupsjokjbpdpg0ymibxv.png)
Substitute the values:
![\[\text{Number of moles} = (5.87 * 10^(22))/(6.022 * 10^(23))\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/3keff2skdhu9d4rxmz3hmvirxqq2bdlp87.png)
Calculating this expression gives:
![\[\text{Number of moles} \approx 0.0976 \, \text{moles}\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/90qhm64dkkrs29o0cjwsm91glk0v6kcri0.png)
So,
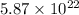 molecules of water is approximately
molecules of water is approximately
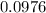 moles of water.
moles of water.