Answer:
Limiting reactant: Cl2.
Max mass of PCl3 = 41.44 g.
P4 leftover = 5.51 g.
Step-by-step explanation:
Hello!
In this case, since the undergoing chemical reaction is:
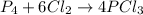
In order to compute the maximum amount of PCl3, it is necessary to compute the grams of this product produced by each reactant, just as shown below, whereas molar masses and mole ratios are used:
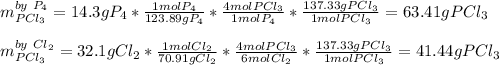
Thus, since chlorine gas yields fewer grams of PCl3 than P4 we infer Cl2 is the limiting reactant and 63.41 grams of PCl3 product are yielded.
Finally, for the excess reactant, we see a difference of 63.41-41.44=21.97g, so we can compute of the leftover of P4 as follows:
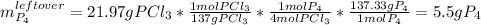
Best regards!