Answer:
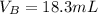 -- Volume of base used
-- Volume of base used
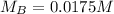 --- Molarity of base
--- Molarity of base
Step-by-step explanation:
Given
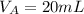 -- Volume of acid used
-- Volume of acid used
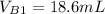 --- Buret Initial reading
--- Buret Initial reading
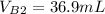 --- Buret Final reading
--- Buret Final reading
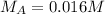 --- Molarity of the acid
--- Molarity of the acid
Solving (a): Volume of base used (VB)
This is calculated by subtracting the initial reading from the final reading of the base buret.
i.e.
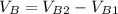
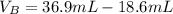
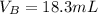
Solving (b): Molarity base (MB)
This is calculated using:
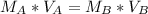
Make MB the subject
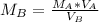
This gives:
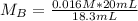
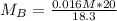
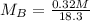
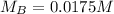
Solving (c): There is no such thing as average molarity