Answer:
There are 10.0 moles of beryllium oxide in a 250 grams sample of the compound.
Step-by-step explanation:
We can calculate the number of moles (η) of BeO as follows:
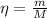
Where:
m: is the mass = 250 g
M: is the molar mass = 25.0116 g/mol
Hence, the number of moles is:
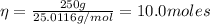
Therefore, there are 10.0 moles of beryllium oxide in a 250 grams sample of the compound.
I hope it helps you!