Answer:
pH=11.
Step-by-step explanation:
Hello!
In this case, since the data is not given, it is possible to use a similar problem like:
"An analytical chemist is titrating 185.0 mL of a 0.7500 M solution of ethylamine(C2HNH2) with a 0.4800 M solution of HNO3.ThepK,of ethylamine is 3.19. Calculate the pH of the base solution after the chemist has added 114.4 mL of the HNO3 solution to it"
Thus, for the reaction:
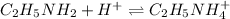
Tt is possible to compute the remaining moles of ethylamine via the following subtraction:
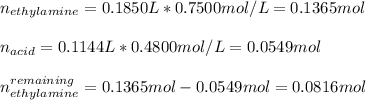
Thus, the concentration of ethylamine in solution is:
![[ethylamine]=(0.0816mol)/(0.1850L+0.1144L)=0.2725M](https://img.qammunity.org/2022/formulas/chemistry/college/1yhfn0741uwododivcq82xtilkxj8hesmi.png)
Now, we can also infer that some salt is formed, and has the following concentration:
![[salt]=(0.0549mol)/(0.1850L+0.1144L)=0.1834M](https://img.qammunity.org/2022/formulas/chemistry/college/8v28cwik7c9w78kry00juq8h5bx2hi0s22.png)
Therefore, we can use the Henderson-Hasselbach equation to compute the resulting pOH first:
![pOH=pKb+log(([salt])/([base]) )\\\\pOH=3.19+log((0.1834M)/(0.2725M))\\\\pOH=3.0](https://img.qammunity.org/2022/formulas/chemistry/college/7j5hs730gc2z85vkvd2rn28tb5linpjuga.png)
Finally, the pH turns out to be:
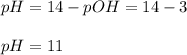
NOTE: keep in mind that if you have different values, you can just change them and follow the very same process here.
Best regards!