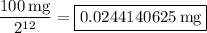Notice that 72 hours is 12 times the half-life of 6 hours. So, the starting amount of 100 mg will decay to half, 12 times. In other words
• after 1 hour, 100 mg decays to 50 mg
• after a total 2 hours, 100 mg decays to 25 mg
• after a total 3 hours, 100 mg decays to 12.5 mg
and so on, so that after a total of 72 hours, the hospital is left with