Solution :
Given that :
The energy of the protons, K.E. = 1.10 keV
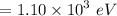
The current produced by the generator is I = 5 mA
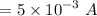
Now
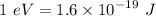
Mass of the proton, m =
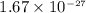 kg
kg
Charge of the proton,

a). Therefore using the formula for K.E. we can find out the velocity of the proton.
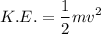
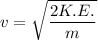
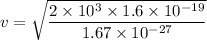
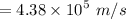
b). We know that the current is :
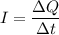
Therefore, the total charge in one second is given by :
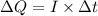
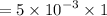
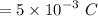
So, the number of protons in this charge is given by :
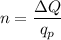
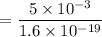
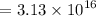 protons
protons