Answer:
a.
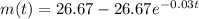 b. 7.44 s
b. 7.44 s
Explanation:
a. If the concentration of salt in the brine entering the tank is 0.2 kg/L, determine the mass of salt in the tank after t min.
Let m(t) be the mass of salt in the tank at any time, t.
Now, since a brine solution flows in at a rate of 4 L/min and has a concentration of 0.2 kg/L, the mass flowing in per minute is m' = 4 L/min × 0.2 kg/L = 0.8 kg/min
Now, the concentration in the tank of volume 100 L at any time, t is m(t)/100 L. Since water flows out at a rate of 3 L/min, the mass flowing out per minute is
m(t)/100 × 3 L/min = 3m(t)/100 kg/min
Now the net rate of change of mass of salt in the tank per minute dm/dt = mass flowing in -mass flowing out
dm/dt = 0.8 kg/min - 3m(t)/100 kg/min
So, dm/dt = 0.8 - 0.03m(t)
The initial mass of salt entering m(0) = 0 kg
dm/dt = 0.8 - 0.03m(t)
separating the variables, we have
dm/[0.8 - 0.03m(t)] = dt
Integrating, we have
∫dm/[0.8 - 0.03m(t)] = ∫dt
-0.03/-0.03 × ∫dm/[0.8 - 0.03m(t)] = ∫dt
1/(-0.03)∫-0.03dm/[0.8 - 0.03m(t)] = ∫dt
-1/0.03㏑[0.8 - 0.03m(t)] = t + C
㏑[0.8 - 0.03m(t)] = -0.03t - 0.03C
㏑[0.8 - 0.03m(t)] = -0.03t + C' (C'= -0.03C)
taking exponents of both sides, we have
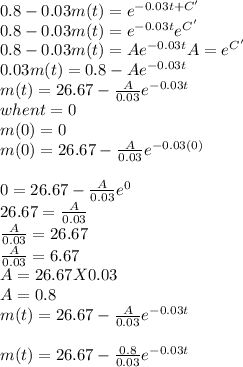
So, the mass of the salt after t min is
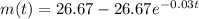
b. When will the concentration of salt in the tank reach 0.1 kg/L?
When the concentration of the salt reaches 0.1 kg/L, m(t) = 0.1 kg/L
Solving the equation for t,
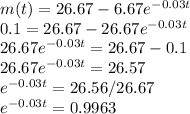
taking natural logarithm of both sides, we have
-0.03t = ㏑0.9963
-0.03t = -0.0038
t = -0.0038/-0.03
t = 0.124 min
t = 0.124 × 60 s
t = 7.44 s