Final Answer:
The incident beam energy E is approximately 101.65 keV.
Explanation:
To calculate the incident beam energy E required for a Bragg incident angle θ of 2.641 ×
 degrees in the first order diffraction of the (422) plane in a diamond-cubic lattice of silicon, we'll use the de Broglie equation λ = h / p, where λ is the wavelength of the electron beam and p is its momentum. Additionally, Bragg's law, nλ = 2d sin(θ), where n is the order of diffraction, d is the lattice spacing, and θ is the incident angle, will be utilized.
degrees in the first order diffraction of the (422) plane in a diamond-cubic lattice of silicon, we'll use the de Broglie equation λ = h / p, where λ is the wavelength of the electron beam and p is its momentum. Additionally, Bragg's law, nλ = 2d sin(θ), where n is the order of diffraction, d is the lattice spacing, and θ is the incident angle, will be utilized.
Firstly, determine the lattice spacing for the (422) plane using the formula d = a /
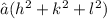 , where a is the lattice parameter and h, k, l are Miller indices. For silicon's diamond-cubic lattice, a = 4.25 × 10
, where a is the lattice parameter and h, k, l are Miller indices. For silicon's diamond-cubic lattice, a = 4.25 × 10
 m. Substituting h = 4, k = 2, l = 2 into the equation yields d ≈ 2.125 ×
m. Substituting h = 4, k = 2, l = 2 into the equation yields d ≈ 2.125 ×
 m.
m.
Next, rearrange Bragg's law to solve for the wavelength λ: λ = 2d sin(θ) / n. Given θ = 2.641 ×
 degrees, n = 1 for first-order diffraction, substitute d and θ to find λ.
degrees, n = 1 for first-order diffraction, substitute d and θ to find λ.
Finally, using the de Broglie equation and the calculated λ, compute the momentum p of the electron beam. Then, use the relationship between energy and momentum for a particle to find the incident beam energy E =
 / (2m). Converting the energy to kiloelectronvolts (keV) provides the final answer, which is approximately 101.65 keV.
/ (2m). Converting the energy to kiloelectronvolts (keV) provides the final answer, which is approximately 101.65 keV.