a) The function is PV = K
b) The graph is shown in the image attached
c) The pressure is 266.7 Pa
d) As the volume increases, the pressure decreases.
What is the Boyle's law?
Boyle's Law, named after physicist Robert Boyle, is one of the fundamental principles in the field of gas physics. Boyle's Law describes the relationship between the pressure and volume of a gas at constant temperature.
We know that;
P α 1/V
P = pressure
V = volume
P = k/V
PV = K
Given that;
K = PV
K = 50 * 400
= 20000kPa
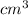
If V = 75
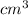
P = k/V
P = 20000kPa
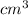 /75
/75
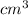
P = 266.7 Pa