Answer:
There would be
 electrons in each sodium atom.
electrons in each sodium atom.
Step-by-step explanation:
Atoms are neutral. In other words, the positive and negative electrical charges in each atom are balanced.
Each atom contains:
- A number of protons, each with an electrical charge of
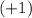 (a positive charge of magnitude
(a positive charge of magnitude
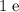 ,)
,) - A number of neutrons, which do not carry any electrical charge, and
- A number of electrons, each with an electrical charge of
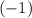 (a negative charge of magnitude
(a negative charge of magnitude
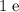 .)
.)
For the positive and negative electrical charges in an atom to balance each other, the number of protons and electrons in that atom should be equal.
For example, the
 protons in the sodium atom in this question would have contributed an electrical charge of
protons in the sodium atom in this question would have contributed an electrical charge of
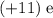 to the atom. Balancing that charge would require an additional electrical charge of
to the atom. Balancing that charge would require an additional electrical charge of
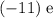 , which corresponds to exactly
, which corresponds to exactly
 electrons.
electrons.