All these reactions (a, b, c, and d) are reduction reactions that can potentially couple with the oxidation of sulfur to form sulfurous acid. Therefore option e. all of the above.
How do we identify the reactions that can be used for oxidation?
 ↔
↔
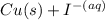 - This reaction is a valid reduction process where CuI is being reduced to Cu and I⁻.
- This reaction is a valid reduction process where CuI is being reduced to Cu and I⁻.
b.
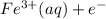 ↔
↔
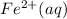 - This reaction is a reduction process where Fe³⁺ is being reduced to Fe²⁺.
- This reaction is a reduction process where Fe³⁺ is being reduced to Fe²⁺.
c.
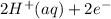 ↔
↔
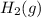 - This is the reduction half-reaction where hydrogen ions (H⁺) gain electrons to form hydrogen gas (H₂).
- This is the reduction half-reaction where hydrogen ions (H⁺) gain electrons to form hydrogen gas (H₂).
d.
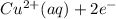 ↔
↔
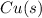 - This is another reduction reaction where Cu²⁺ ions gain electrons to form copper metal.
- This is another reduction reaction where Cu²⁺ ions gain electrons to form copper metal.