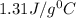Answer: The specific heat capacity of metal is
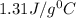
Step-by-step explanation:
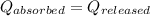
As we know that,
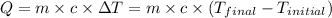
![m_1* c* (T_(final)-T_1)=-[m_2* c* (T_(final)-T_2)]](https://img.qammunity.org/2022/formulas/chemistry/high-school/jh7i56dwqx67c73qu0jayph5ocsl5wgsqt.png)
where,
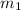 = mass of metal = 35 g
= mass of metal = 35 g
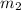 = mass of water = 220 g
= mass of water = 220 g
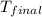 = final temperature =
= final temperature =
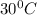
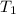 = temperature of metal =
= temperature of metal =
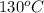
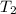 = temperature of water =
= temperature of water =
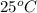
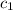 = specific heat of metal = ?
= specific heat of metal = ?
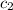 = specific heat of water =
= specific heat of water =
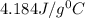
Now put all the given values in equation (1), we get
![m_1* c_1* (T_(final)-T_1)=-[m_2* c_2* (T_(final)-T_2)]](https://img.qammunity.org/2022/formulas/chemistry/high-school/2iwsj4v7xth00ba9r1k0icno5vnyyj4swj.png)
![35* c_1* (30-130)^0C=-[220g* 4.184* (30-25)]](https://img.qammunity.org/2022/formulas/chemistry/high-school/lfhupx3eqeotr128hbevqgbb49taus0xxw.png)
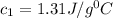
Therefore, the specific heat capacity of metal is