The binding energy of the Ni is obtained as 1.4 *
 J.
J.
What is binding energy?
Binding energy refers to the energy required to disassemble a system of particles into individual components or, conversely, the energy released when these components come together.
Mass of protons = 28 * 1.007275
= 18.2037 u
Mass of neutrons = 30(1.008666)
= 30.25998 u
Total mass = 18.2037 u + 30.25998 u
= 48.46368 u
Difference in mass = 57.935348 u - 48.46368 u
= 9.471668 u
We know that;
ΔE = m
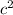
= 9.471668 u * 1.66*
 kg * (3 *
kg * (3 *
 m/s
m/s
= 1.4 *
 J
J