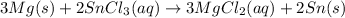Answer:
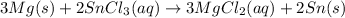
Step-by-step explanation:
Single replacement reaction is a chemical reaction in which more reactive element displaces the less reactive element from its salt solution.
A more reactive element is one which can easily lose or gain electrons as compared to the other element.
As Magnesium is more reactive than tin, it can easily displace tin from its salt solution
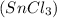 and form magnesium chloride and tin in elemental form.
and form magnesium chloride and tin in elemental form.
The balanced chemical equation is: