Answer:
[OH⁻] = 0.002 M
Step-by-step explanation:
The definition of pOH is similar to the definition of pH:
This means that with a given pOH value, the calculation of [OH⁻] is a matter of algebra:
To remove the logarithm we increase 10 by the power of the pOH:
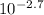 =[OH⁻]
=[OH⁻]