Answer:
Q = 2627.25 J
Step-by-step explanation:
Given that,
The mass of water, m = 28.7 g
The temperature of the water to increase from 11.3 °C to 33.2 °C
The specific heat of water is 4.18 J/gºC
The heat absorbed by the water is given by :
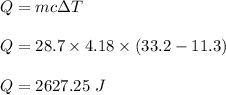
So, 2627.25 J of heat is absorbed by the water.